Vein Visualization Leader Sponsors Symposium at American Organization for Nursing Leadership (AONL)
MEDFORD, NY–(BUSINESS WIRE)–AccuVein Inc.the world leader in near-infrared (NIR) vein visualization, announced today that on Wednesday, April 13 AONL at the Convention Center of San Antonio, Texas, Henry B. Gonzalez.
The symposium is titled “Reclaim Nursing Time and Improve Patient Care with NIR Visualization”. Participants will learn how to reduce work stress, reclaim care time, and improve patient care by incorporating NIR vein visualization into their peripheral IV (PIV) site assessment and clinical protocol. The session will be chaired by Gregory J. Schears, MD, and Thomas Hopkins, MD, MBA, CPE, who will jointly summarize evidence demonstrating the value of NIR and best practice tips to improve patient satisfaction and improve provider resilience for use both adults and pediatric patients will be discussed.
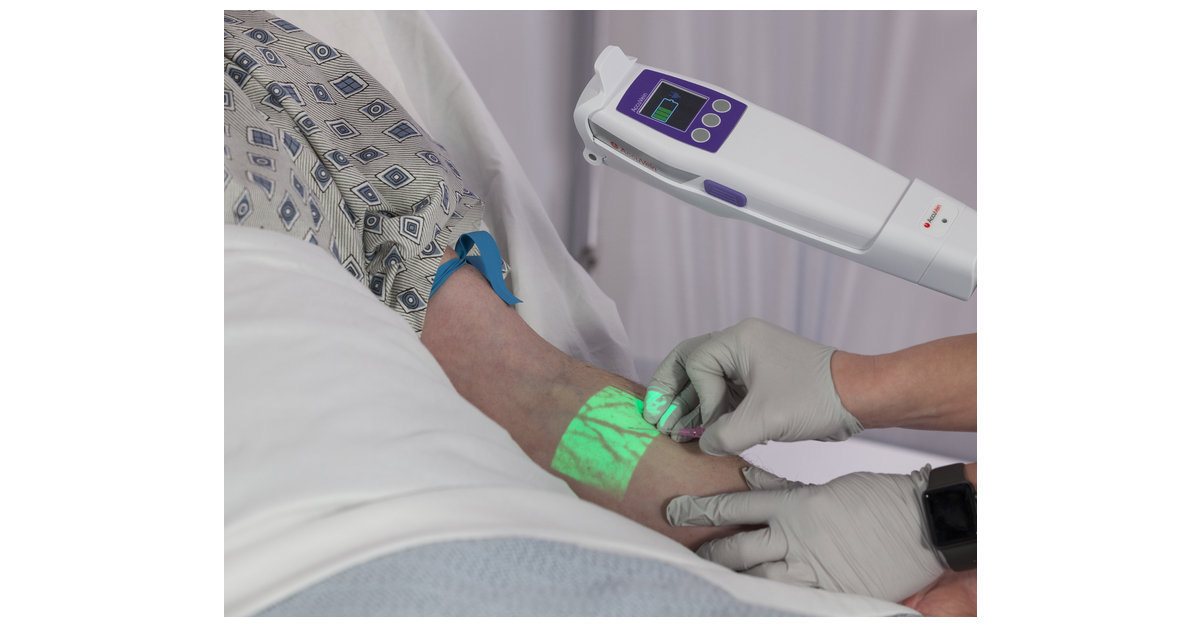
AONL visitors can also visit AccuVein’s booth (#1042) Monday, April 11 – Wednesday, April 13, where AccuVein will demonstrate its latest vein visualization device, the AV500, and showcase its robust training/education program.
“We recognize that the COVID-19 pandemic has placed additional strain on the nursing profession, exposing nurses to conditions that threaten their health, well-being and overall ability to provide clinical care,” said Thomas Hopkins, chief medical officer at AccuVein Inc. “We are very proud and committed to offering our healthcare heroes the latest advances in NIR vein visualization technology, which can help limit their susceptibility to infectious diseases while improving PIV efficiency and reclaiming valuable care time. In addition, we now offer bespoke training and education support to address and alleviate stress and anxiety in caregiving, and help with the onboarding of new employees,” added Hopkins.
Recent studies show that AccuVein-guided PIV assessment and placement can reduce procedure time by 78%* and increase the caregiver’s ability to successfully place peripheral intravenous catheters (PIVC) the first time in place by 92%*, leading to leads to improved PIV efficiency and effectiveness. In addition, AccuVein can also reduce the risk of cross-contamination to other workers by reducing the need for escalation calls to the IV team or to a more costly ultrasound-guided IV insertion procedure by 45%*. These study results suggest that AccuVein is helping nurses limit their exposure to COVID-19. Click here for more clinical evidence supporting the benefits of AccuVein here.
Up to 90% of patients receive IV therapy at some point during their hospital stay, making it the most commonly performed invasive medical procedure. Although routine, venipuncture can be technically demanding. If multiple venous access attempts are required, this is associated with increased patient morbidity and increased use of staff and medical supplies.
Additionally the Infusion Nursing Society (INS) 2021 Infusion Therapy Standards Clearly linking NIR vein visualization to improving outcomes and overall patient experience, further supporting its application in a clinical setting. The standards state: “It is important that vascular visualization technology is employed to increase the success of introducing the most appropriate, least invasive vascular access device (VAD), minimize the need to escalate to an unnecessary, more invasive device, and reduce insertion. associated complications.”
About AccuVein Inc.
AccuVein Inc. is a pioneer in developing novel medical technologies that enable healthcare professionals to improve vein assessment and maintain vascular health. The company has developed the only patented, eye-safe, laser-based device, the AV500, that uses near-infrared (NIR) technology to help physicians see superficial veins, valves, and bifurcations to make more informed decisions and perform precise needle placements. / Catheter Placement. AccuVein is used in more than 5,000 facilities and is available in over 130 countries worldwide. To see a demonstration of the AccuVein AV500 in action and to learn more, please visit here.
*Data on hand

Comments are closed.